电喷雾电离
编辑电喷雾电离(ESI)是一种用于质谱分析的技术,利用电喷雾产生离子,其中向液体施加高电压以产生气溶胶。它在使大分子产生离子方面特别有用,因为其克服了使大分子电离时被碎裂的倾向。电喷雾电离不同于其他电离过程(例如基质辅助激光解吸/电离(MALDI)),其可以产生多电荷离子,有效地扩大分析仪的测量范围,并可蛋白质及其相关多肽片段实验中探测到的kDa-MDa数量级的电离大分子。[1][2]
使用电喷雾质谱技术的质谱被称为电喷雾电离质谱,或者被称为电喷雾质谱。电喷雾电离是一种所谓的“软电离”技术,因为几乎没有碎片,因此比较有利。因为分子离子(或者更准确地说是伪分子离子)可以被检测到,然而从从简单质谱只能获得非常少的结构信息。其可以通过将电喷雾电离与串联质谱联用来克服。电喷雾电离的另一个重要优点是液相信息可以保留在气相中。
电喷雾电离技术由山下正道和约翰·芬于1984年首次报道。二者因[3]电喷雾电离用于生物大分子分析的发展[4]获得了2002年诺贝尔化学奖。[5]芬恩博士使用的原始仪器之一后来在宾夕法尼亚州费城的科学历史研究所展出。
目录编辑
- 4.1 主要文章:液相色谱-质谱
- 4.2 毛细管电泳质谱
- 4.3 气相中非共价相互作用的应用
- 5参考文献
1 历史编辑
2 电离机制编辑
目标分析物的液滴通过电喷雾,[14]分散成细气溶胶。因为离子形成涉及大量溶剂蒸发(也称为去溶剂化),电喷雾电离的典型溶剂是通过将水与挥发性有机化合物(例如甲醇[15]乙腈)混合来制备的。为了减小初始液滴尺寸,通常向溶液中加入增加电导率的化合物(如乙酸)。这些物质还提供质子源来促进电离过程。除了电喷雾源的高温之外,大流量电喷雾还可以受益于加热的惰性气体如氮气或二氧化碳的雾化。[16]气溶胶通过带有大约3000伏电位差的毛细管后被进入到质谱仪的第一级真空,毛细管可以被加热以帮助从带电液滴中进一步蒸发溶剂。溶剂从带电液滴蒸发,直到达到瑞利极限时变得不稳定。此时,液滴变形,因为液滴尺寸不断减小,从而出现电荷静电排斥的力强于将液滴表面张力的现象。[17]在这一点上,液滴经历库仑裂变,从而原始液滴“分裂”,产生许多更小、更稳定的液滴。新液滴经历去溶剂化和随后的库仑破裂。裂变过程中,液滴损失了一小部分质量(1.0-2.3%),同时也损失了相对大部分电荷(10-18%)。[18]
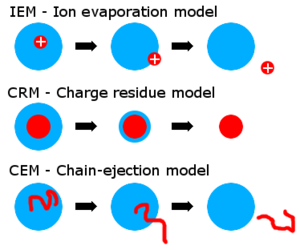
解释气相离子最终产生的主要理论有两种:离子蒸发模型(IEM)和电荷残留模型(CRM)。IEM理论认为,当液滴达到一定半径时,液滴表面的场强变得足够大,从而导致溶剂化离子解吸。[19][20] CRMR认为电喷雾液滴经历蒸发和分裂循环,最终导致含有大约一个分析物离子的子代液滴。[21]气相离子在剩余的溶剂分子蒸发后形成,留下分析物和液滴携带的电荷。
大量证据直接或间接表明,小离子(来自小分子)通过离子蒸发机制释放到气相中,[[20][21][22]而较大离子(例如来自折叠蛋白)通过带电残留物机制形成。 [23][24][25]
现已经提出了第三种模型,该模型是组合电荷剩余电场诱发粒子释放的模式。[26]另一个被称为链弹射模型(CEM)的模型是针对无序聚合物(未折叠蛋白质)提出的。[27]
质谱观察到的离子可以是通过添加氢阳离子产生的准分子离子,表示为[M + H]+,或另一种阳离子,例如钠离子,[M +钠]+,或去除氢核,[M-H]。经常观察到多电荷离子,如[M + nH]n+离子。对于大分子来说,可以有许多电荷态,从而产生一个特征电荷态包络。所有这些都是偶电子离子种类:电子(单独的)不被添加或移除,不同于其他一些电离源。分析物有时涉及电化学过程,导致质谱中相应峰的移动。这种效应在使用电喷雾对铜、银和金等贵金属的直接电离中得到证明。[28]
3 改进与变异编辑
在低流速下运行的电喷雾产生更小的初始液滴,这确保了更高的电离效率。1993年盖尔和理查德·史密斯报告指出,使用较低的流速,灵敏度可以显著提高,降至200 nL/min。[29] 1994年,两个研究小组将在低流速下工作的电喷雾命名为微电喷雾。当电喷雾以300–800 nL/min的速度运行时,埃米特和卡普里奥利证明了高效液相色谱-质谱分析方法的的性能提高。[30] 威尔姆和曼恩证明,约25 nL/min的毛细管流可以维持通过将玻璃毛细管拉至几微米而制造的极其细的电喷雾毛细管中。[31] 后者于1996年更名为纳米电喷雾。[32][33] 目前纳米喷雾的名称也用于通过泵推动以低流速供给的电喷雾模式中,[34] 其不仅仅用于自供给电喷雾。虽然电喷雾、微喷雾和纳米电喷雾可能没有明确的流速范围,[35]但 研究了“离子释放前液滴分裂过程可知液滴的缩小进程快于离子的释放过程。[35] 其结果是通过对照其他三组的数据以及[36][37][38] 测量不同流速下的[Ba2++ Ba+]/[BaB+]信号强度比而得出的。
冷喷雾电离是电喷雾的一种形式,其中含有样品的溶液被通过一个小的冷毛细管(10-80°C )进入电场,产生冷带电液滴的细雾。[39] 该方法的应用包括分析脆弱分子和不能用常规电喷雾电离研究的蛋白间相互作用。
电喷雾电离也可以在低至25托的压力下实现,理查德·史密斯及其同事开发出两级离子漏斗界面的纳米电喷雾压力电离模式。[40]由于使用了有助于将离子限制和转移到质谱仪低压区域的离子漏斗,自旋实施提供了更高的灵敏度。纳米电喷雾发射器由细毛细管制成,小孔径约为1-3微米。为了获得足够的导电性,该毛细管通常溅射涂覆有导电材料,例如金。纳米电喷雾电离只消耗几微升样品并形成更小的液滴。[41]低压操作对于低流速特别有效,其中较小的电喷雾液滴尺寸允许实现有效的去溶剂化和离子形成。因此,研究人员后来能够证明,将离子从液相转移到气相中作为离子,并通过双离子漏斗界面转移到质谱仪,总电离利用效率超过50%。[42]
3.1 环境电离
在环境电离中,离子的形成发生在质谱仪之外,无需样品制备。[43][44][45] 电喷雾形成的离子是由环境离子源提供的。
解吸电喷雾电离是一种环境电离技术,其中溶剂电喷雾直接针对样品。[46][47] 通过对样品施加电压,电喷雾被吸引到表面。样品化合物被提取到溶剂中,溶剂再次以高电荷液滴的形式雾化,蒸发形成高电荷离子。电离后,离子进入质谱仪的大气压界面。DESI允许样品在大气压下的环境电离,样品制备很少。
萃取电喷雾电离是一种喷雾型环境电离方法,使用两种方法合并喷雾,其中一种由电喷雾产生。[44]
基于激光的电喷雾环境电离模式是一个两步过程,其中使用脉冲激光从样品中解吸或烧蚀材料表面,材料末流与电喷雾相互作用产生离子。[44]对于环境电离,样品材料沉积在电喷雾附近的靶上。激光从表面喷出的样品中解吸或烧蚀材料,然后电离粒子进入产生高电荷离子的电喷雾中。这类型模式包括有电喷雾激光解吸电离、基质辅助激光解吸电喷雾电离和激光烧蚀电喷雾电离。
静电喷雾电离(ESTASI)涉及位于平坦或多孔表面或微通道内部的样品分析。将含有分析物的液滴沉积在样品区域,向样品区域施加脉冲高压。当静电压力大于表面张力时,液滴和离子被喷射。
二次电喷雾电离(SESI)是一种喷雾型环境电离方法,其中充电离子通过电喷雾产生。当这些离子与它们碰撞时,会给气相中的蒸汽分子充电。
在纸喷雾电离中,样品被施加到一张纸上,添加溶剂,并且高电压被施加到纸上,然后产生离子。
4 应用程序编辑
4.1 主要文章:液相色谱-质谱
电喷雾电离是液相色谱和质谱联用的离子源。分析可以在线进行,将液相色谱柱洗脱的液体直接送至电喷雾模组,其操作也可以离线进行,收集馏分后在纳米电喷雾质谱装置中进行分析。在电喷雾质谱的众多操作参数中,[51]研究表明:[52]电喷雾电压已被确定为电喷雾液相色谱/质谱梯度洗脱中需要考虑的一个重要参数。[53]各种溶剂组合物[55](如TFA[54][56)或醋酸铵,[55]或增压试剂,[55][56][57][58] 或衍生基团[59]或喷涂条件[60]对电喷雾-液相色谱质谱和/或纳米电喷雾-质谱都具有一定影响。[61]
4.2 毛细管电泳质谱
毛细管电泳-质谱分析是由理查德·史密斯和其太平洋西北国家实验室的同事开发并获得专利的电喷雾质谱方法,它在分析非常小的生物和化学化合物混合物时具有优势,甚至可以用于研究到单个生物细胞方面的探究中,具有广泛的用途。
4.3 气相中非共价相互作用的应用
电喷雾电离也被用于研究非共价气相相互作用。电喷雾过程被认为能够将液相非共价复合物转移到气相中,而不破坏非共价相互作用。当用电喷雾质谱或纳米电喷雾质谱研究配体底物复合物时,发现[55][62] 非特异性相互作用的问题。[63]一个有趣的例子是研究酶和作为酶抑制剂的药物之间的相互作用。电喷雾电离方式被用于[64][65][66] stat 6和抑制剂[66][67][68] 之间的竞争实验,其模式也演变为筛选潜在新药靶标分子的。
参考文献
- [1]
^Ho, CS; Chan MHM; Cheung RCK; Law LK; Lit LCW; Ng KF; Suen MWM; Tai HL (February 2003). "Electrospray Ionisation Mass Spectrometry: Principles and Clinical Applications". Clin Biochem Rev. 24 (1): 3–12. PMC 1853331. PMID 18568044..
- [2]
^Pitt, James J (February 2009). "Principles and Applications of Liquid Chromatography-Mass Spectrometry in Clinical Biochemistry". Clin Biochem Rev. 30 (1): 19–34. PMC 2643089. PMID 19224008..
- [3]
^Yamashita, Masamichi; Fenn, John B. (September 1984). "Electrospray ion source. Another variation on the free-jet theme". The Journal of Physical Chemistry. 88 (20): 4451–4459. doi:10.1021/j150664a002..
- [4]
^Fenn, J. B.; Mann, M.; Meng, C. K.; Wong, S. F.; Whitehouse, C. M. (1989). "Electrospray ionization for mass spectrometry of large biomolecules". Science. 246 (4926): 64–71. Bibcode:1989Sci...246...64F. CiteSeerX 10.1.1.522.9458. doi:10.1126/science.2675315. PMID 2675315..
- [5]
^Markides, K; Gräslund, A. "Advanced information on the Nobel Prize in Chemistry 2002" (PDF)..
- [6]
^Rayleigh, L. (1882). "On the Equilibrium of Liquid Conducting Masses charged with Electricity". Philosophical Magazine. 14 (87): 184–186. doi:10.1080/14786448208628425..
- [7]
^Zeleny, J. (1914). "The electrical discharge from liquid points, and a hydrostatic method of measuring the electric intensity at their surfaces". Physical Review. 3 (2): 69–91. Bibcode:1914PhRv....3...69Z. doi:10.1103/PhysRev.3.69..
- [8]
^Wilson, C. T.; G. I Taylor (1925). "The bursting of soap bubbles in a uniform electric field". Proc. Cambridge Philos. Soc. 22 (5): 728. Bibcode:1925PCPS...22..728W. doi:10.1017/S0305004100009609..
- [9]
^Nolan, J. J. (1926). "Universal scaling laws for the disintegration of electrified drops". Proc. R. Ir. Acad. A. 37: 28..
- [10]
^Macky, W. A. (October 1, 1931). "Some Investigations on the Deformation and Breaking of Water Drops in Strong Electric Fields". Proceedings of the Royal Society A. 133 (822): 565–587. Bibcode:1931RSPSA.133..565M. doi:10.1098/rspa.1931.0168..
- [11]
^Geoffrey Taylor (1964). "Disintegration of Water Droplets in an Electric Field". Proceedings of the Royal Society A. 280 (1382): 383–397. Bibcode:1964RSPSA.280..383T. doi:10.1098/rspa.1964.0151. JSTOR 2415876..
- [12]
^Birendra N. Pramanik; A.K. Ganguly; Michael L. Gross (28 February 2002). Applied Electrospray Mass Spectrometry: Practical Spectroscopy Series. CRC Press. pp. 4–. ISBN 978-0-8247-4419-9..
- [13]
^"Press Release: The Nobel Prize in Chemistry 2002". The Nobel Foundation. 2002-10-09. Retrieved 2011-04-02..
- [14]
^Pozniak BP, Cole RB (2007). "Current Measurements within the Electrospray Emitter". JASMS. 18 (4): 737–748. doi:10.1016/j.jasms.2006.11.012. PMID 17257852..
- [15]
^Olumee; et al. (1998). "Droplet Dynamics Changes in Electrostatic Sprays of Methanol-Water Mixtures". J. Phys. Chem. A. 102 (46): 9154–9160. Bibcode:1998JPCA..102.9154O. CiteSeerX 10.1.1.661.5000. doi:10.1021/jp982027z..
- [16]
^Fernández De La Mora J (2007). "The Fluid Dynamics of Taylor Cones". Annual Review of Fluid Mechanics. 39 (1): 217–243. Bibcode:2007AnRFM..39..217F. doi:10.1146/annurev.fluid.39.050905.110159..
- [17]
^Cole, Richard B (2010). Electrospray and MALDI Mass Spectrometry: Fundamentals, Instrumentation, Practicalities, and Biological Applications (2 ed.). Wiley. p. 4. ISBN 978-0471741077..
- [18]
^Li KY, Tu H, Ray AK (April 2005). "Charge limits on droplets during evaporation". Langmuir. 21 (9): 3786–94. doi:10.1021/la047973n. PMID 15835938..
- [19]
^Iribarne JV, Thomson BA (1976). "On the evaporation of small ions from charged droplets". Journal of Chemical Physics. 64 (6): 2287–2294. Bibcode:1976JChPh..64.2287I. doi:10.1063/1.432536..
- [20]
^Nguyen S, Fenn JB (January 2007). "Gas-phase ions of solute species from charged droplets of solutions". Proc. Natl. Acad. Sci. USA. 104 (4): 1111–7. Bibcode:2007PNAS..104.1111N. doi:10.1073/pnas.0609969104. PMC 1783130. PMID 17213314..
- [21]
^Dole M, Mack LL, Hines RL, Mobley RC, Ferguson LD, Alice MB (1968). "Molecular Beams of Macroions". Journal of Chemical Physics. 49 (5): 2240–2249. Bibcode:1968JChPh..49.2240D. doi:10.1063/1.1670391..
- [22]
^de la Mora Fernandez (2000). "Electrospray ionization of large multiply charged species proceeds via Dole's charged residue mechanism". Analytica Chimica Acta. 406: 93–104. doi:10.1016/S0003-2670(99)00601-7. An evaluation of the electric field on the drop surface at the point when it just ceases to be spherical (yet carries the total ion charge z) indicates that small PEG ions may be formed by ion evaporation. The break observed in the charge distribution may perhaps mean that the shift from the Dole to the ion evaporation mechanism arises at m(unintelligible)104Attention! The article once absorbed some rude copy-and-pastes, so this may actually stand for 104[需要解释], though this inference is highly hypothetical..
- [23]
^de la Mora Fernandez (2000). "Electrospray ionization of large multiply charged species proceeds via Dole's charged residue mechanism". Analytica Chimica Acta. 406: 93–104. doi:10.1016/S0003-2670(99)00601-7..
- [24]
^de la Mora Fernandez (2000). "Electrospray ionization of large multiply charged species proceeds via Dole's charged residue mechanism". Analytica Chimica Acta. 406: 93–104. doi:10.1016/S0003-2670(99)00601-7. For most published data examined, zmax is between 65% and 110% of zR, providing strong support in favor of Dole’s charged residue mechanism, at least for masses from 3.3 kD up to 1.4 MD. Other large but less compact ions from proteins and linear chains of polyethylene glycols (PEGs) have zmax values considerably larger than zR, apparently implying that they also formas charged residues, though from non-spherical drops held together by the polymer backbone..
- [25]
^de la Mora Fernandez (2000). "Electrospray ionization of large multiply charged species proceeds via Dole's charged residue mechanism". Analytica Chimica Acta. 406: 93–104. doi:10.1016/S0003-2670(99)00601-7. The data do show a nearly discontinuous jump in the observed m/z for a mass somewhere between 20,000 and 50,000, and it is tempting to conclude that this is due to a corresponding transition where the ionization mechanism shifts from one type to the other. This would correspond to a critical value of z in the vicinity of 50, with a corresponding electric field of 2.6 V/nm. Of course, this is entirely hypothetical, and there is yet no compelling evidence of any kind indicating that an ion with as many as 30 charges can be formed by field evaporation..
- [26]
^Hogan CJ, Carroll JA, Rohrs HW, Biswas P, Gross ML (January 2009). "Combined charged residue-field emission model of macromolecular electrospray ionization". Anal. Chem. 81 (1): 369–77. doi:10.1021/ac8016532. PMC 2613577. PMID 19117463..
- [27]
^Unraveling the Mechanism of Electrospray Ionization. Lars Konermann, Elias Ahadi, Antony D. Rodriguez and Siavash Vahidi, Anal. Chem., 2013, 85 (1), pages 2–9, doi:10.1021/ac302789c.
- [28]
^Li, Anyin; Luo, Qingjie; Park, So-Jung; Cooks, R. Graham (2014). "Synthesis and Catalytic Reactions of Nanoparticles formed by Electrospray Ionization of Coinage Metals". Angewandte Chemie International Edition. 53 (12): 3147–3150. doi:10.1002/anie.201309193. ISSN 1433-7851. PMID 24554582..
- [29]
^Gale DC, Smith RD (1993). "Small Volume and Low Flow Rate Electrospray Ionization Mass Spectrometry for Aqueous Samples". Rapid Commun. Mass Spectrom. 7 (11): 1017–1021. Bibcode:1993RCMS....7.1017G. doi:10.1002/rcm.1290071111..
- [30]
^Emmett MR, Caprioli RM (1994). "Micro-electrospray mass spectrometry: ultra-high-sensitivity analysis of peptides and proteins". J. Am. Soc. Mass Spectrom. 5 (7): 605–613. doi:10.1016/1044-0305(94)85001-1..
- [31]
^Wilm MS, Mann M (1994). "Electrospray and Taylor-Cone theory, Dole's beam of macromolecules at last?". Int. J. Mass Spectrom. Ion Process. 136 (2–3): 167–180. Bibcode:1994IJMSI.136..167W. doi:10.1016/0168-1176(94)04024-9..
- [32]
^Wilm M, Mann M (1996). "Analytical properties of the nanoelectrospray ion source". Anal. Chem. 68 (1): 1–8. doi:10.1021/ac9509519. PMID 8779426..
- [33]
^Gibson; Mugo, Samuel M.; Oleschuk, Richard D.; et al. (2009). "Nanoelectrospray emitters: Trends and perspective". Mass Spectrometry Reviews. 28 (6): 918–936. Bibcode:2009MSRv...28..918G. doi:10.1002/mas.20248. PMID 19479726..
- [34]
^Page JS, Marginean I, Baker ES, Kelly RT, Tang K, Smith RD (December 2009). "Biases in ion transmission through an electrospray ionization-mass spectrometry capillary inlet". J. Am. Soc. Mass Spectrom. 20 (12): 2265–72. doi:10.1016/j.jasms.2009.08.018. PMC 2861838. PMID 19815425..
- [35]
^Schmidt A, Karas M, Dülcks T (May 2003). "Effect of different solution flow rates on analyte ion signals in nano-ESI MS, or: when does ESI turn into nano-ESI?". J. Am. Soc. Mass Spectrom. 14 (5): 492–500. doi:10.1016/S1044-0305(03)00128-4. PMID 12745218..
- [36]
^Wilm M. S.; Mann M. (1994). "Electrospray and Taylor-Cone Theory, Dole's Beam of Macromolecules at Last?". Int. J. Mass Spectrom. Ion Process. 136 (2–3): 167–180. Bibcode:1994IJMSI.136..167W. doi:10.1016/0168-1176(94)04024-9..
- [37]
^Fernandez de la Mora J., Loscertales I. G. (2006). "The Current Emitted by Highly Conducting Taylor Cones". J. Fluid Mech. 260: 155–184. Bibcode:1994JFM...260..155D. doi:10.1017/S0022112094003472..
- [38]
^Pfeifer RJ, Hendricks (1968). "Parametric Studies of Electrohydrodynamic Spraying". AIAA J. 6 (3): 496–502. Bibcode:1968AIAAJ...6..496H. doi:10.2514/3.4525..
- [39]
^RSC Chemical Methods Ontology, Cold-spray ionisation mass spectrometry.
- [40]
^Page JS, Tang K, Kelly RT, Smith RD (2008). "A subambient pressure ionization with nanoelectrospray (SPIN) source and interface for improved sensitivity in mass spectrometry". Analytical Chemistry. 80 (5): 1800–1805. doi:10.1021/ac702354b. PMC 2516344. PMID 18237189..
- [41]
^Karas, M.; Bahr, U.; Dülcks, T. (2000-03-01). "Nano-electrospray ionization mass spectrometry: addressing analytical problems beyond routine". Fresenius' Journal of Analytical Chemistry (in 英语). 366 (6–7): 669–676. doi:10.1007/s002160051561. ISSN 0937-0633..
- [42]
^I. Marginean; J. S. Page; A. V. Tolmachev; K. Tang; R. D. Smith (2010). "Achieving 50% Ionization Efficiency in Subambient Pressure Ionization with Nanoelectrospray". Analytical Chemistry. 82 (22): 9344–9349. doi:10.1021/ac1019123. PMC 2982749. PMID 21028835..
- [43]
^Cooks, R. Graham; Ouyang, Zheng; Takats, Zoltan; Wiseman, Justin M. (2006). "Ambient Mass Spectrometry". Science. 311 (5767): 1566–70. Bibcode:2006Sci...311.1566C. doi:10.1126/science.1119426. PMID 16543450..
- [44]
^Monge, María Eugenia; Harris, Glenn A.; Dwivedi, Prabha; Fernández, Facundo M. (2013). "Mass Spectrometry: Recent Advances in Direct Open Air Surface Sampling/Ionization". Chemical Reviews. 113 (4): 2269–2308. doi:10.1021/cr300309q. ISSN 0009-2665. PMID 23301684..
- [45]
^Huang, Min-Zong; Yuan, Cheng-Hui; Cheng, Sy-Chyi; Cho, Yi-Tzu; Shiea, Jentaie (2010). "Ambient Ionization Mass Spectrometry". Annual Review of Analytical Chemistry. 3 (1): 43–65. Bibcode:2010ARAC....3...43H. doi:10.1146/annurev.anchem.111808.073702. ISSN 1936-1327. PMID 20636033..
- [46]
^Z. Takáts; J.M. Wiseman; B. Gologan; R.G. Cooks (2004). "Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization". Science. 306 (5695): 471–473. Bibcode:2004Sci...306..471T. doi:10.1126/science.1104404. PMID 15486296..
- [47]
^Takáts Z, Wiseman JM, Cooks RG (2005). "Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms and applications in forensics, chemistry, and biology". Journal of Mass Spectrometry. 40 (10): 1261–75. Bibcode:2005JMSp...40.1261T. doi:10.1002/jms.922. PMID 16237663..
- [48]
^Konermann, L; Douglas, DJ (1998). "Equilibrium unfolding of proteins monitored by electrospray ionization mass spectrometry: Distinguishing two-state from multi-state transitions". Rapid Communications in Mass Spectrometry. 12 (8): 435–442. Bibcode:1998RCMS...12..435K. doi:10.1002/(SICI)1097-0231(19980430)12:8<435::AID-RCM181>3.0.CO;2-F. PMID 9586231..
- [49]
^Nemes; Goyal, Samita; Vertes, Akos; et al. (2008). "Conformational and Noncovalent Complexation Changes in Proteins during Electrospray Ionization". Analytical Chemistry. 80 (2): 387–395. doi:10.1021/ac0714359. PMID 18081323..
- [50]
^Sobott; Robinson (2004). "Characterising electrosprayed biomolecules using tandem-MS—the noncovalent GroEL chaperonin assembly". International Journal of Mass Spectrometry. 236 (1–3): 25–32. Bibcode:2004IJMSp.236...25S. doi:10.1016/j.ijms.2004.05.010..
- [51]
^for proteins: Vaidyanathan S.; Kell D.B.; Goodacre R. (2004). "Selective detection of proteins in mixtures using electrospray ionization mass spectrometry: influence of instrumental settings and implications for proteomics". Analytical Chemistry. 76 (17): 5024–5032. doi:10.1021/ac049684. PMID 15373437..
- [52]
^Iavarone; Jurchen, John C.; Williams, Evan R.; et al. (2000). "Effects of solvent on the maximum charge state and charge state distribution of protein ions produced by electrospray ionization". JASMS. 11 (11): 976–985. doi:10.1016/S1044-0305(00)00169-0. PMC 1414794. PMID 11073261..
- [53]
^Marginean I, Kelly RT, Moore RJ, Prior DC, LaMarche BL, Tang K, Smith RD (April 2009). "Selection of the optimum electrospray voltage for gradient elution LC-MS measurements". J. Am. Soc. Mass Spectrom. 20 (4): 682–8. doi:10.1016/j.jasms.2008.12.004. PMC 2692488. PMID 19196520..
- [54]
^Garcia (2005). "The effect of the mobile phase additives on sensitivity in the analysis of peptides and proteins by high-performance liquid chromatography–electrospray mass spectrometry". Journal of Chromatography B. 825 (2): 111–123. doi:10.1016/j.jchromb.2005.03.041. PMID 16213445..
- [55]
^Kebarle P, Verkerk UH (2009). "Electrospray: from ions in solution to ions in the gas phase, what we know now". Mass Spectrom Rev. 28 (6): 898–917. Bibcode:2009MSRv...28..898K. doi:10.1002/mas.20247. PMID 19551695..
- [56]
^Lomeli SH, Peng IX, Yin S, Loo RR, Loo JA (January 2010). "New reagents for increasing ESI multiple charging of proteins and protein complexes". J. Am. Soc. Mass Spectrom. 21 (1): 127–31. doi:10.1016/j.jasms.2009.09.014. PMC 2821426. PMID 19854660..
- [57]
^Lomeli SH, Yin S, Ogorzalek Loo RR, Loo JA (April 2009). "Increasing charge while preserving noncovalent protein complexes for ESI-MS". J. Am. Soc. Mass Spectrom. 20 (4): 593–6. doi:10.1016/j.jasms.2008.11.013. PMC 2789282. PMID 19101165..
- [58]
^Yin S, Loo JA (March 2011). "Top-Down Mass Spectrometry of Supercharged Native Protein-Ligand Complexes". Int J Mass Spectrom. 300 (2–3): 118–122. Bibcode:2011IJMSp.300..118Y. doi:10.1016/j.ijms.2010.06.032. PMC 3076692. PMID 21499519..
- [59]
^Krusemark CJ, Frey BL, Belshaw PJ, Smith LM (September 2009). "Modifying the charge state distribution of proteins in electrospray ionization mass spectrometry by chemical derivatization". J. Am. Soc. Mass Spectrom. 20 (9): 1617–25. doi:10.1016/j.jasms.2009.04.017. PMC 2776692. PMID 19481956..
- [60]
^Nemes P, Goyal S, Vertes A (January 2008). "Conformational and noncovalent complexation changes in proteins during electrospray ionization". Anal. Chem. 80 (2): 387–95. doi:10.1021/ac0714359. PMID 18081323..
- [61]
^Ramanathan R, Zhong R, Blumenkrantz N, Chowdhury SK, Alton KB (October 2007). "Response normalized liquid chromatography nanospray ionization mass spectrometry". J. Am. Soc. Mass Spectrom. 18 (10): 1891–9. doi:10.1016/j.jasms.2007.07.022. PMID 17766144..
- [62]
^Gabelica V, Vreuls C, Filée P, Duval V, Joris B, Pauw ED (2002). "Advantages and drawbacks of nanospray for studying noncovalent protein-DNA complexes by mass spectrometry". Rapid Commun. Mass Spectrom. 16 (18): 1723–8. Bibcode:2002RCMS...16.1723G. doi:10.1002/rcm.776. PMID 12207359..
- [63]
^Daubenfeld T, Bouin AP, van der Rest G (September 2006). "A deconvolution method for the separation of specific versus nonspecific interactions in noncovalent protein-ligand complexes analyzed by ESI-FT-ICR mass spectrometry". J. Am. Soc. Mass Spectrom. 17 (9): 1239–48. doi:10.1016/j.jasms.2006.05.005. PMID 16793278..
- [64]
^Rosu F, De Pauw E, Gabelica V (July 2008). "Electrospray mass spectrometry to study drug-nucleic acids interactions". Biochimie. 90 (7): 1074–87. doi:10.1016/j.biochi.2008.01.005. PMID 18261993..
- [65]
^Wortmann A, Jecklin MC, Touboul D, Badertscher M, Zenobi R (May 2008). "Binding constant determination of high-affinity protein-ligand complexes by electrospray ionization mass spectrometry and ligand competition". J Mass Spectrom. 43 (5): 600–8. Bibcode:2008JMSp...43..600W. doi:10.1002/jms.1355. PMID 18074334..
- [66]
^Jecklin MC, Touboul D, Bovet C, Wortmann A, Zenobi R (March 2008). "Which electrospray-based ionization method best reflects protein-ligand interactions found in solution? a comparison of ESI, nanoESI, and ESSI for the determination of dissociation constants with mass spectrometry". J. Am. Soc. Mass Spectrom. 19 (3): 332–43. doi:10.1016/j.jasms.2007.11.007. PMID 18083584..
- [67]
^Touboul D, Maillard L, Grässlin A, Moumne R, Seitz M, Robinson J, Zenobi R (February 2009). "How to deal with weak interactions in noncovalent complexes analyzed by electrospray mass spectrometry: cyclopeptidic inhibitors of the nuclear receptor coactivator 1-STAT6". J. Am. Soc. Mass Spectrom. 20 (2): 303–11. doi:10.1016/j.jasms.2008.10.008. PMID 18996720..
- [68]
^Czuczy N, Katona M, Takats Z (February 2009). "Selective detection of specific protein-ligand complexes by electrosonic spray-precursor ion scan tandem mass spectrometry". J. Am. Soc. Mass Spectrom. 20 (2): 227–37. doi:10.1016/j.jasms.2008.09.010. PMID 18976932..
暂无