苯并三唑
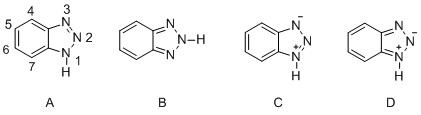
编辑1 结构编辑
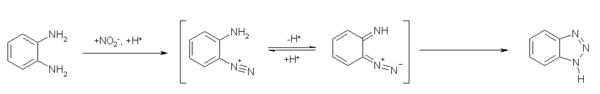
2 合成编辑
3 应用编辑
苯并三唑以其广泛的用途而闻名。它已经被用作照相乳剂的抑制剂和银的分析测定试剂。更重要的是,它已被广泛用作大气和水下的缓蚀剂。此外,其衍生物及其作为药物前体的有效性也引起了人们的关注。除了上面提到的所有应用之外,BTA还可以用作防冻剂、加热和冷却系统、液压流体和气相抑制剂。[6]
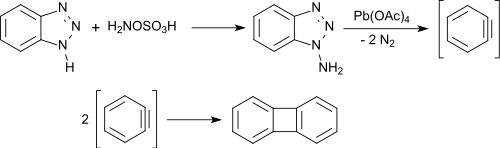
以苯并三唑为原料,通过羟胺-O-磺酸的氨基化反应可以方便地制备联苯和苄。主要产物1-氨基苯并三唑通过用乙酸铅(IV价)氧化以几乎定量的产率生成苯并三唑,乙酸铅氧化后迅速二聚生成联苯烯,产率很高。[6]
3.1 腐蚀抑制剂
苯并三唑是铜及其合金的有效缓蚀剂,可防止不良的表面反应发生。众所周知,当铜浸入含有苯并三唑的溶液中时,会形成由铜和苯并三唑之间的络合物组成的钝化层。钝化层不溶于水和多种有机溶液。钝化层的厚度和防腐蚀效率之间呈正相关。[7] BTA可以用于保护、特别是用于防止青铜的腐蚀。铜-苯系物复合体的确切结构存在争议,科学家们对此提出了很多假设。
3.2 药物前体
苯并三唑衍生物具有化学和生物特性,在制药工业中具有广泛的用途。苯并三唑衍生物可用作许多蛋白质的促进剂。例如,沃氯唑和阿立必利对不同的蛋白质具有有效的抑制作用,苯并三唑酯已被报道用作严重急性呼吸综合征(SARS)3CL蛋白酶的灭活剂。该方法不仅适用于杂环化,而且对于适用于小碳环体系的多核烃。[8]
参考文献
- [1]
^Katritzky, A. R.; Rachwal S.; Hitchings G. J. (14 January 1991). "Benzotriazole: A novel synthetic auxiliary". Tetrahedron. 47 (16–17): 2683–2732. doi:10.1016/S0040-4020(01)87080-0..
- [2]
^Katritzky, A. R. "Adventures with Benzotriazole" (PDF). Lecture presented at various locations in 2002. Florida Center for Heterocyclic CompoundsFor. Archived from the original (PDF) on 26 April 2012. Retrieved 23 November 2011..
- [3]
^Robert A. Smiley "Phenylene- and Toluenediamines" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a19_405.
- [4]
^Claudio, M. P.; Pereira, Helio A.; Stefani, Karla P.; Guzen, Aline T. G. (29 August 2006). "Improved Synthesis of Benzotriazoles and 1-Acylbenzotriazoles by Ultrasound Irradiation" (PDF). Letters in Organic Chemistry. 4 (31): 43–46. doi:10.1002/chin.200731104. Retrieved 23 November 2011.[永久失效连结].
- [5]
^"Benzotriazole - Chemical Supplier Distributor Chemceed"..
- [6]
^Sease, Catherine (May 1978). "Benzotriazole: A Review for Conservators". Studies in Conservation. 2. 23 (2): 76–85. doi:10.2307/1505798. JSTOR 1505798..
- [7]
^Finšgar, M.; Milošev I. (11 March 2010). "Inhibition of copper corrosion by 1,2,3-benzotriazole: A review". Corrosion Science. 52 (9): 2737–2749. doi:10.1016/j.corsci.2010.05.002..
- [8]
^Kale, Raju R.; Virendra Prasad; Prabhu P. Mohapatra; Vinod K. Tiwari (6 March 2010). "Recent developments in benzotriazole methodology for construction of pharmacologically important heterocyclic skeletons". Monatsh Chemistry. 141 (11): 1159–1182. doi:10.1007/s00706-010-0378-1..
- [9]
^Giger, W; Schaffner, C; Kohler, HP (2006). "Benzotriazole and tolyltriazole as aquatic contaminants. 1. Input and occurrence in rivers and lakes". Environmental Science & Technology. 40 (23): 7186–92. doi:10.1021/es061565j. PMID 17180965..
暂无