丁子香酚
编辑1 现代用途编辑
丁子香酚被应用于香水、香料和精油中。它也被用作局部防腐剂和麻醉剂。[9][10]丁子香酚可以与氧化锌结合形成氧化锌丁子香酚,该物质在牙科中具有整形和修复的应用。对于因拔牙并发症而出现干槽症的人,用带有丁子香酚氧化锌糊剂的碘仿纱布包裹干槽可有效减轻急性疼痛。[11] 丁子香酚氧化锌糊剂也用于根管封闭。[12]
丁子香酚是对多种雄性兰花蜂有吸引力的众多化合物之一,这些蜜蜂显然收集这种化学物质来合成信息素;丁子香酚通常被用作诱饵来吸引和采集这些蜜蜂来进行研究。[13] 它也吸引雌性黄守瓜。[14]最近发现丁子香酚和异丁子香酚这些花卉挥发性气味化合物是由手参属的单一种类酶催化的,而编码这种酶的基因是迄今为止这些物种中第一个功能特征基因。
丁香油作为麻醉剂越来越受欢迎,用于以研究和管理为目的对观赏鱼和野生鱼进行采样。[15][16] 在容易获得的地方,它提供了一种人道的方法,通过直接过量使用丁子香酚或在过量使用丁子香酚前诱导睡眠来对患病的鱼实施安乐死。[17]
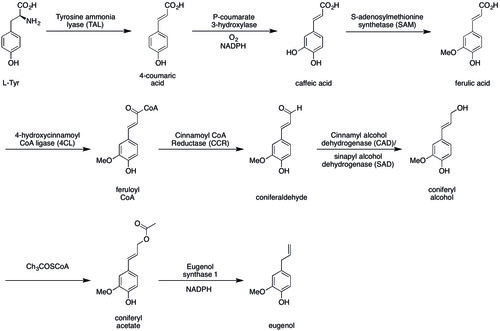
2 生物合成编辑
3 毒性编辑
4 过敏反应编辑
参考文献
- [1]
^National Center for Biotechnology Information. PubChem Compound Database; CID=3314, https://pubchem.ncbi.nlm.nih.gov/compound/3314 (accessed Feb. 26, 2019)..
- [2]
^"Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum L.) Alston" (PDF). Bangladesh Council of Scientific and Industrial Research BCSIR Laboratories. 4: 451–454..
- [3]
^Mallavarapu, Gopal R.; Ramesh, S.; Chandrasekhara, R. S.; Rajeswara Rao, B. R.; Kaul, P. N.; Bhattacharya, A. K. (1995). "Investigation of the essential oil of cinnamon leaf grown at Bangalore and Hyderabad". Flavour and Fragrance Journal. 10 (4): 239–242. doi:10.1002/ffj.2730100403..
- [4]
^Yield and Oil Composition of 38 Basil (Ocimum basilicum L.) Accessions Grown in Mississippi Archived 15 10月 2010 at the Wayback Machine.
- [5]
^"Typical G.C. for bay leaf oil". Thegoodscentscompany.com. Retrieved 2014-04-27..
- [6]
^Barnes, J.; Anderson, L. A.; Phillipson, J. D. (2007) [1996]. Herbal Medicines (PDF) (3rd ed.). London: Pharmaceutical Press. ISBN 978-0-85369-623-0..
- [7]
^"Human Metabolome Database: Showing metabocard for Eugenol (HMDB0005809)". www.hmdb.ca. Retrieved 2018-07-01..
- [8]
^Cortés-Rojas DF, de Souza CR, Oliveira WP (February 2014). "Clove (Syzygium aromaticum): a precious spice". Asian Pac J Trop Biomed. 4 (2): 90–6. doi:10.1016/S2221-1691(14)60215-X. PMC 3819475. PMID 25182278..
- [9]
^Sell, AB; Carlini, EA (1976). "Anesthetic action of methyleugenol and other eugenol derivatives". Pharmacology. 14 (4): 367–77. doi:10.1159/000136617. PMID 935250..
- [10]
^Jadhav, B. K.; Khandelwal, K. R.; Ketkar, A. R.; Pisal, S. S. (February 2004). "Formulation and evaluation of mucoadhesive tablets containing eugenol for the treatment of periodontal diseases". Drug Development and Industrial Pharmacy. 30 (2): 195–203. doi:10.1081/DDC-120028715. PMID 15089054..
- [11]
^Tarakji B, Saleh LA, Umair A, Azzeghaiby SN, Hanouneh S (April 2015). "Systemic review of dry socket: aetiology, treatment, and prevention". J Clin Diagn Res. 9 (4): ZE10–3. doi:10.7860/JCDR/2015/12422.5840. PMC 4437177. PMID 26023661..
- [12]
^Ferracane, Jack L. (2001). Materials in Dentistry: Principles and Applications (2nd ed.). Lippincott, Williams & Wilkins. ISBN 978-0-7817-2733-4..
- [13]
^Schiestl, F. P.; Roubik, D. W. (January 2003). "Odor Compound Detection in Male Euglossine Bees". Journal of Chemical Ecology. 29 (1): 253–257. doi:10.1023/A:1021932131526. PMID 12647866. Archived from the original on 3 February 2013. Retrieved 4 February 2009..
- [14]
^"Cucumber Beetles: Organic and Biorational Integrated Pest Management (Summary)". Attra.ncat.org. 2013-08-05. Retrieved 2014-04-27..
- [15]
^Anesthesia, Analgesia, and Surgery in Pet Fish. Atlantic Coast Veterinary Conference. 2001. Retrieved 2014-03-17..
- [16]
^Grush, J.; Noakes, D. L. G.; Moccia, R. D. (February 2004). "The Efficacy of Clove Oil As An Anesthetic for the Zebrafish". Zebrafish. 1 (1): 46–53. doi:10.1089/154585404774101671. PMID 18248205..
- [17]
^Monks, Neale (2009-04-02). "Aquarium Fish Euthanasia" (PDF). Fish Channel. Retrieved 2010-12-07..
- [18]
^Dewick, P. M. (2009). Medicinal Natural Products. John Wiley & Sons. doi:10.1002/9780470742761. ISBN 9780470742761..
- [19]
^Harakava, R. (2005). "Genes encoding enzymes of the lignin biosynthesis pathway in Eucalyptus". Genet. Mol. Biol. 28: 601–607..
- [20]
^Thompson, D. C.; Barhoumi, R.; Burghardt, R. C. (1998). "Comparative toxicity of eugenol and its quinone methide metabolite in cultured liver cells using kinetic fluorescence bioassays". Toxicology and Applied Pharmacology. 149 (1): 55–63. doi:10.1006/taap.1997.8348. PMID 9512727..
- [21]
^Fujisawa, S.; Atsumi, T.; Kadoma, Y.; Sakagami, H. (2002). "Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity". Toxicology. 177 (1): 39–54. doi:10.1016/S0300-483X(02)00194-4. PMID 12126794..
- [22]
^"Eugenol Oil Overdose". The New York Times..
- [23]
^Hartnoll, G.; Moore, D.; Douek, D. (1993). "Near fatal ingestion of oil of cloves". Archives of Disease in Childhood. 69 (3): 392–393. doi:10.1136/adc.69.3.392. PMC 1029532. PMID 8215554..
- [24]
^"IFRA". www.ifraorg.org. Archived from the original on 2011-12-30..
- [25]
^"Cropwatch Claims Victory Regarding "26 Allergens" Legislation" (PDF). www.leffingwell.com. Retrieved 2014-04-27..
- [26]
^Schmalz, Gottfried; Bindslev, Dorthe Arenholt (2008-10-10). Biocompatibility of Dental Materials. ISBN 9783540777823. Retrieved 2014-04-27..
- [27]
^Bruynzeel, Derk P. (2014). "Balsam of Peru (Myroxylon pereirae)". Management of Positive Patch Test Reactions. Springer. pp. 53–55. doi:10.1007/978-3-642-55706-4_11. ISBN 978-3-540-44347-6..
- [28]
^Pathak, S. B.; Niranjan, K.; Padh, H.; Rajani, M. (2004). "TLC Densitometric Method for the Quantification of Eugenol and Gallic Acid in Clove". Chromatographia. 60 (3–4): 241–244. doi:10.1365/s10337-004-0373-y..
- [29]
^Bullerman, L. B.; Lieu, F. Y.; Seier, S. A. (July 1977). "Inhibition of growth and aflatoxin production by cinnamon and clove oils. Cinnamic aldehyde and eugenol". Journal of Food Science. 42 (4): 1107–1109. doi:10.1111/j.1365-2621.1977.tb12677.x..
- [30]
^Lee, Kwang-Geun; Shibamoto, Takayuki (2001). "Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. et Perry]". Food Chemistry. 74 (4): 443–448. doi:10.1016/S0308-8146(01)00161-3..
- [31]
^Kreydiyyeh, S. I.; Usta, J.; Copti, R. (2000). "Effect of cinnamon, clove and some of their constituents on the Na+-K+-ATPase activity and alanine absorption in the rat jejunum". Food and Chemical Toxicology. 38 (9): 755–762. doi:10.1016/S0278-6915(00)00073-9. PMID 10930696..
- [32]
^Dighe, V. V.; Gursale, A. A.; Sane, R. T.; Menon, S.; Patel, P. H. (2005). "Quantitative Determination of Eugenol from Cinnamomum tamala Nees and Eberm. Leaf Powder and Polyherbal Formulation Using Reverse Phase Liquid Chromatography". Chromatographia. 61 (9–10): 443–446. doi:10.1365/s10337-005-0527-6..
- [33]
^Bennett, A.; Stamford, I. F.; Tavares, I. A.; Jacobs, S.; Capasso, F.; Mascolo, N.; Autore, G.; Romano, V.; Di Carlo, G. (1988). "The biological activity of eugenol, a major constituent of nutmeg (..Myristica fragrans..): Studies on prostaglandins, the intestine and other tissues". Phytotherapy Research. 2 (3): 124–130. doi:10.1002/ptr.2650020305..
- [34]
^Johnson, C. B.; Kirby, J.; Naxakis, G.; Pearson, S. (1999). "Substantial UV-B-mediated induction of essential oils in sweet basil (Ocimum basilicum L.)". Phytochemistry. 51 (4): 507–510. doi:10.1016/S0031-9422(98)00767-5..
- [35]
^Gupta, A. K.; Schauvinhold, I.; Pichersky, E.; Schiestl, F. P. (2014). "Eugenol synthase genes in floral scent variation in Gymnadenia species". Functional & Integrative Genomics. 14 (4): 779–788. doi:10.1007/s10142-014-0397-9. PMID 25239559..
- [36]
^Ize-Ludlow, D.; Ragone, S.; Bruck, I. S.; Bernstein, J. N.; Duchowny, M.; Peña, B. M. (2004). "Neurotoxicities in Infants Seen With the Consumption of Star Anise Tea". Pediatrics. 114 (5): e653–e656. doi:10.1542/peds.2004-0058. PMID 15492355..
- [37]
^"Lemon balm". University of Maryland Medical Center. Retrieved 2010-12-07..
暂无